Read Time: 8 mins
On Christmas day, 1957, a baby was born to an employee of Chemie Gruenthal, a German pharmaceutical company. This baby was the first of nearly 10,000 infants who faced a tragedy that would change the way that the world tested, marketed and sold drugs forever.
In 1956, Chemie Gruenthal developed a miracle drug. It was an effective tranquillizer, with limited to no addictive properties. It was non-toxic, with a lethal dose in animals that was so large that it was seen as one of the safest drugs known to man. As an added bonus, the drug helped with morning sickness. As a result, it was marketed to thousands of women in America and Europe and it had 37 different brand names. The drug was called thalidomide.
Teratogens
By 1960, it was obvious there were problems. Mothers who had been offered the drug in early pregnancy were giving birth to babies who hadn’t developed in the normal way. The most common effect was shortened limbs, but the drug impacted the eyes, brain and auditory system too. Drugs that impact limb development are known as teratogens.
Despite a number of warnings which were apparently ignored by the company, the drug remained in circulation until 1961, when a doctor by the name of William McBride published an open letter in The Lancet medical journal. Eventually, Thalidomide was banned, but not before it affected nearly 10,000 infants of whom 40% tragically died in childbirth.
The mirror image – isomerism
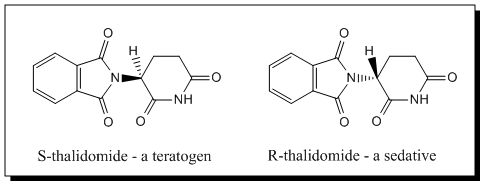
Drugs are made up of different compounds, bound together in molecules. The type and number of individual chemicals in a molecule are what make different drugs different and change the effect they have on the body. However, chemical makeup is not the only thing that can impact the effect a drug has. In some cases, certain drug compounds can have exactly the same chemical composition, but their structure (the way that the chemicals are arranged in space) is different. In other drugs, the structure is the same, but the specific bonds between the chemicals are different. This is known as isomerism and is the reason why Thalidomide was so dangerous.
Thalidomide has 2 isomers. They readily interchange in the body which means that it’s impossible to isolate one from the other. The first, Thalidomide (R) is completely safe. It is responsible for the tranquillizing effect and the anti-nausea. The other, Thalidomide (S) is teratogenic and responsible for the impact on infants from 1957 – 1962.
The Overhaul
The impact that thalidomide had on the pharmaceutical industry was extreme. In the USA, prior to 1962, drug companies were allowed to market drugs if the FDA (Food and Drug Administration) hadn’t acted in 60 days. That meant that the FDA had less than 2 months to assess the safety of any given drug and if it missed the deadline, it was open season. That all changed in 1962. The legislation introduced into law amendments which seem so fundamental by today’s standards, it’s almost lunacy:
For example, the amendments included a law that said drug companies now had to prove that a drug worked before being put to market. Incredibly, this was not the case prior to 1962. Companies had to use proper clinical trials and make sure that labels included potential side effects. In the UK, the scandal led to the instruction of prescription-only medicines versus over-the-counter.
The Thalidomide scandal was borne out of lax regulation and poor testing and controls. The company should have understood more about the different isomers, and they should have been more thorough in their testing. The regulatory changes were important but too little too late for the thousands of families impacted by the drugs.
But isomers have also played another role for drug companies. This time, one that helps them turn a tidy profit.
Heartburn
For anyone too young or blessed to not have experienced heartburn, it’s awful. Imagine swallowing red-hot metal and letting it steep in your insides. It’s caused by stomach acid essentially overspilling into the digestive tract and the oesophagus. Luckily there are lots of ways to help it, ranging back to something as simple as chalk. One of the more effective drugs is called omeprazole.
In 1979, omeprazole was invented. It helps heartburn by stopping the secretion of stomach acid. It does it very well. In 2001, the patent for Omezaprole ran out. When a patent on a drug runs out, it becomes generic which means that anyone can manufacture it. This the foundation for Amazon’s new RxPass initiative whereby it provides access in the USA to cheap generic medication. Naturally, this was a problem for the company that owned the patent because it was a very popular drug that made a lot of money.
So the drug company looked at its options and it found something interesting. Omeprazole was made up of 2 different isomers. Unlike Thalidomide, both of these isomers were completely safe.
So, the company did some tests and found that they could isolate one of these isomers. Technically, this would count as a different drug, so they patented it. The “new” drug is called Nexium and it’s one of the most popular heartburn medications on the market at the time of writing. The problem is, at best, it’s as effective as omeprazole. At worst, it’s slightly less effective. Why is this important? Look at how much they cost:
- Nexium (newly patented): £7.90 for 7 tabs or £1.13 per unit
- Omeprazole (generic medication): £11.95 for 56 units or £0.21p per unit
That’s insane. For every 1 Nexium, you can get 5.65 tablets of Omezaprole. And they are basically the same drug.
Lessons learned
Pharmaceutical companies are businesses and it’s worth keeping in mind that the only reason that drug companies now have to prove their drugs work through clinical trials is that they were compelled to by a tragedy that killed thousands and dramatically impacted the lives of thousands more. Thalidomide is still used today, to treat a type of cancer called myeloma. It’s subject to serious restrictions including mandated use of contraception.
Just because these safeguards are in place, this doesn’t protect consumers from other commercial tricks and marketing spin that are both legal according to regulatory bodies and encouraged by big pharma’s shareholders. The sleight of hand we see with Omeprazole and Nexium are testament to that.
The books “Bad Science” and “Bad Pharma” written by Ben Goldacre reference many instances where companies have tried their very best to circumnavigate these rules in order to sell more drugs and increase their profit. They can do this in multiple ways – withholding test data, cherry-picking results or burying negative trials. A great example of this was the drug “Tamiflu”. This was supposed to reduce the chances of death for people who contracted the flu. The UK government spent millions on the drug. The problem was, nearly half of the clinical trials for Tamiflu had been withheld. If you withhold half the results of something, you can persuade anyone of anything. If I withhold half of the results of a coin toss, I can persuade you I have a double-headed coin.
The above is not advice or a recommendation in any way. Speak to your doctor or physician before making any changes designed to impact your health. But it quite literally pays to be cautious, especially when it’s legal for companies to sell what is more or less the same drug for a 500% uplift.
To understand more about Bad Science, you can watch Ben Goldacre’s TED talk here.
There are similarities between the tricks that big pharma play and the tricks that used to be used by big tobacco and more recently, vape manufacturers.